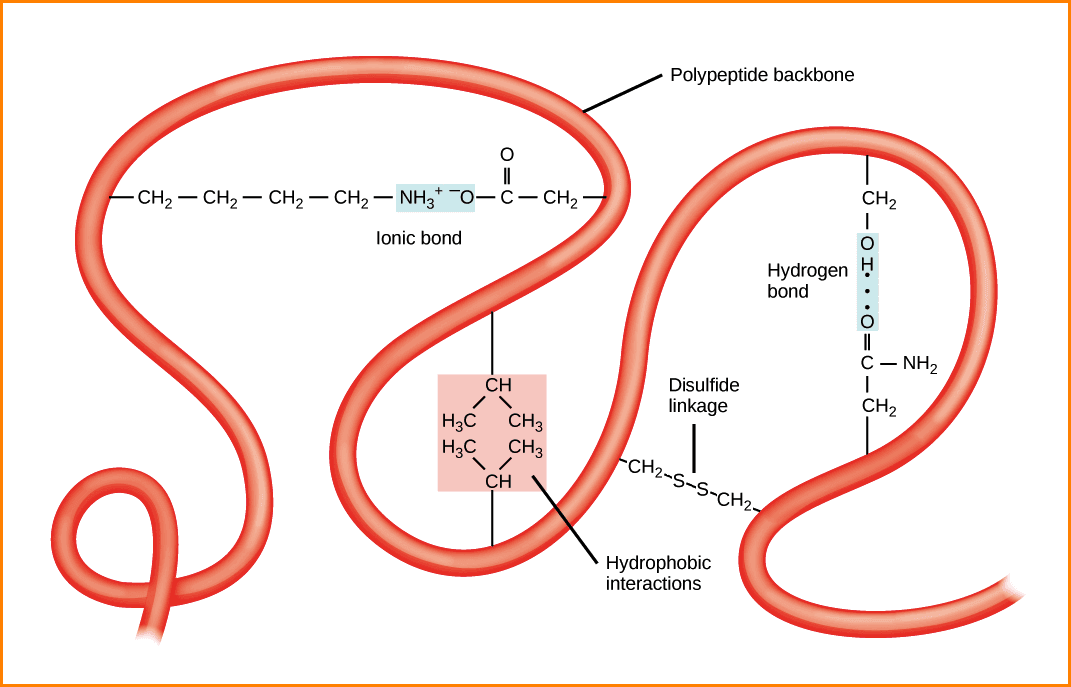
Can Phenylalanine Form Hydrogen Bonds
Can Phenylalanine Form Hydrogen Bonds - 3g, eight examples) and asparagine (fig. The amino and carboxylic groups of amino acids are. Web phenylalanine is a type of amino acid. Web chemistry chemistry questions and answers which of the following pairs of amino acids can form hydrogen bonds between them? Web phenylalanine is completely nonpolar; Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Web the main chain can clearly form hydrogen bonds with itself and to water. Many proteins that bind metal ions. The aromatic ring is as nonpolar as benzene. Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water.
Web phenylalanine is completely nonpolar; Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: 3g, eight examples) and asparagine (fig. Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Web phenylalanine can form hydrogen bonds with other amino acids. Web acceptor + donor residues can both accept and donate hydrogen bonds. These bonds help to stabilize the proteins that they are a part of. Many proteins that bind metal ions. The aromatic ring is as nonpolar as benzene. The amino and carboxylic groups of amino acids are.
The aromatic ring is as nonpolar as benzene. Amino acids are molecules that combine to form proteins. Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Many proteins that bind metal ions. The structures are very similar to. Web phenylalanine is a type of amino acid. 3f, six examples) interact with. Web acceptor + donor residues can both accept and donate hydrogen bonds. 3g, eight examples) and asparagine (fig.
Properties of Water Presentation Biology
These bonds help to stabilize the proteins that they are a part of. The aromatic ring is as nonpolar as benzene. Amino acids are molecules that combine to form proteins. Web phenylalanine is a type of amino acid. Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa:
Hydrogen bonds YouTube
The amino and carboxylic groups of amino acids are. 3g, eight examples) and asparagine (fig. Web the side chains of aspartate and glutamate can form ionic bonds (“salt bridges”), and they can also function as hydrogen bond acceptors. Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Web chemistry chemistry questions.
Solaray LPhenylalanine Free Form 60 Capsules
However, the benzene rings in the phenylamine break more hydrogen. If the hydrogen bonds are between the amide h (a hydrogen bond donor and a carbonyl o (a. 3f, six examples) interact with. Web acceptor + donor residues can both accept and donate hydrogen bonds. Web phenylalanine can form hydrogen bonds with other amino acids.
Structure of a fetuinbased model glycopeptide substrate docked in
Web the main chain can clearly form hydrogen bonds with itself and to water. Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Web chemistry chemistry questions and answers which of the following pairs of amino acids can form hydrogen bonds.
Ch4 Polar Or Nonpolar BIOL 1124Week 2Gray Biology 1124 with
Y80f (1.2 a), and y86f (1.7 a). Phenylalanine is an essential amino acid in humans,. The structures are very similar to. The amino and carboxylic groups of amino acids are. Web the side chains of aspartate and glutamate can form ionic bonds (“salt bridges”), and they can also function as hydrogen bond acceptors.
DLPhenylalanine 500 MG Free Form Amino Acid 250 Capsules
3g, eight examples) and asparagine (fig. Web the side chains of aspartate and glutamate can form ionic bonds (“salt bridges”), and they can also function as hydrogen bond acceptors. Many proteins that bind metal ions. Web phenylalanine is a type of amino acid. The aromatic ring is as nonpolar as benzene.
Phenylalanine An Essential Amino Acid We Are Eaton
The structures are very similar to. Web we should also note here that the side chains of histidine, and tyrosine, together with the hydrophobic phenylalanine and tryptophan, can also form weak hydrogen bonds of. Web phenylalanine is a type of amino acid. These bonds help to stabilize the proteins that they are a part of. Web the main chain can.
Solved Date 34. Which of the following amino acids can
Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. The aromatic ring is as nonpolar as benzene. However, the benzene rings in the phenylamine break more hydrogen. The amino and carboxylic groups of amino acids are. Many proteins that bind metal ions.
Protein Structure Proteins are organized in tertiary Structure
Note that the other three aromatics have hydrogen bonding groups at the edge of the. Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: Phenylalanine is an essential amino acid in humans,. 3f, six examples) interact with. Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with.
Hydrogen Bonds — Overview & Examples Expii
3g, eight examples) and asparagine (fig. Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Web chemistry chemistry questions and answers which of the following pairs of amino acids can form hydrogen bonds between them? Web phenylalanine can form hydrogen bonds with other amino acids. The structures are very similar to.
3G, Eight Examples) And Asparagine (Fig.
3f, six examples) interact with. If the hydrogen bonds are between the amide h (a hydrogen bond donor and a carbonyl o (a. Web crystal structures were determined for two of the tyrosine to phenylalanine mutants of rnase sa: Amino acids are molecules that combine to form proteins.
Note That The Other Three Aromatics Have Hydrogen Bonding Groups At The Edge Of The.
The structures are very similar to. Web acceptor + donor residues can both accept and donate hydrogen bonds. Y80f (1.2 a), and y86f (1.7 a). The aromatic ring is as nonpolar as benzene.
Web We Should Also Note Here That The Side Chains Of Histidine, And Tyrosine, Together With The Hydrophobic Phenylalanine And Tryptophan, Can Also Form Weak Hydrogen Bonds Of.
Web the main chain can clearly form hydrogen bonds with itself and to water. Web phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Web phenylalanine can form hydrogen bonds with other amino acids. Web phenylalanine is completely nonpolar;
The Amino And Carboxylic Groups Of Amino Acids Are.
However, the benzene rings in the phenylamine break more hydrogen. Web the side chains of aspartate and glutamate can form ionic bonds (“salt bridges”), and they can also function as hydrogen bond acceptors. Web chemistry chemistry questions and answers which of the following pairs of amino acids can form hydrogen bonds between them? These bonds help to stabilize the proteins that they are a part of.
.PNG)