How Many Covalent Bonds Can Chlorine Form
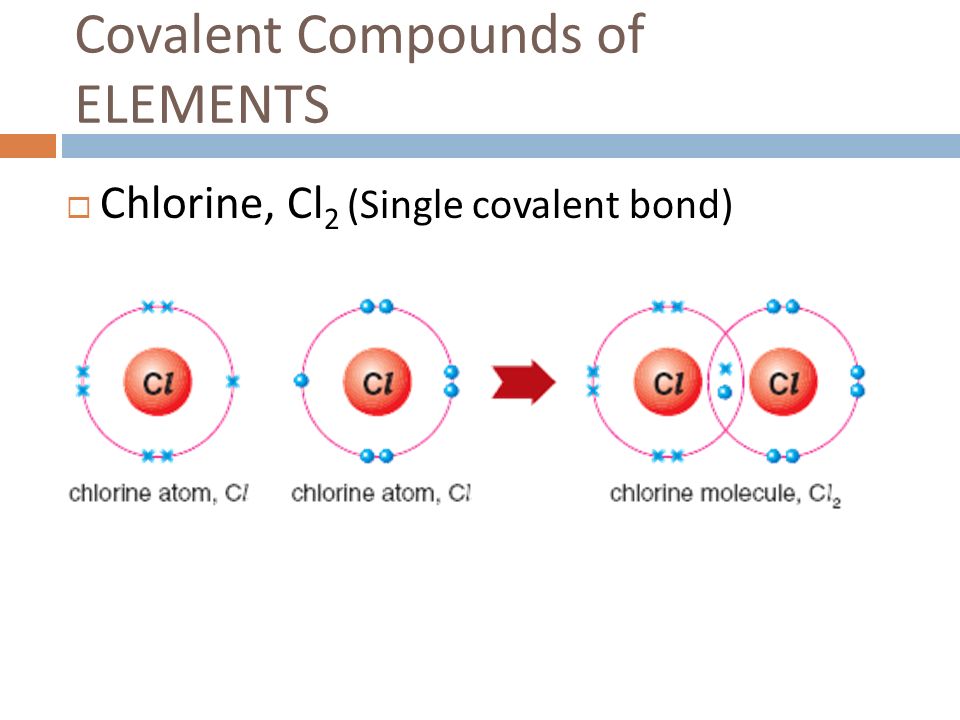
How Many Covalent Bonds Can Chlorine Form - The halogens all have the general electron configuration ns2np5, giving them seven valence. Web how many covalent bonds does chlorine form? Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for an octet. The order of bonding, and so the valence state of cl in cloxxx−, x > 1 c l o x x x −, x > 1 compounds is very debatable. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. This is called covalent bonding. And group 7a form one bond. The more electrons that are shared between two. Web usually each atom contributes one electron to the shared pair of electrons. If it shares one of those with another chlorine atom (and the other one with the first), they can both.
The more electrons that are shared between two. Web typically, the atoms of group 4a form 4 covalent bonds; If it shares one of those with another chlorine atom (and the other one with the first), they can both. 2cl2 + 2h2o → 4hcl +o2 (2). Web how many covalent bonds can chlorine form? Web cl2 +h2o → hocl + hcl (1) (1) c l 2 + h 2 o → h o c l + h c l at the boiling temperature of water, chlorine decomposes water: Group 5a form 3 bonds; 1 pm = 1 × 10 −12 m). The order of bonding, and so the valence state of cl in cloxxx−, x > 1 c l o x x x −, x > 1 compounds is very debatable. Web we refer to this as a pure covalent bond.
Web typically, the atoms of group 4a form 4 covalent bonds; Web usually each atom contributes one electron to the shared pair of electrons. Web how many covalent bonds does chlorine form? Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for an octet. The more electrons that are shared between two. Web in chlorine an electron pair is shared between the two atoms in cl 2. Web the number refers to the number of bonds each of the element makes: Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. In the case of cl 2, each atom starts off. And group 7a form one bond.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web the number refers to the number of bonds each of the element makes: 2cl2 + 2h2o → 4hcl +o2 (2). Web how many covalent bonds can chlorine form? They end of sharing 6 electrons between the. Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for.
Ionic Bonds BOOKSTRONAUTS
Web we refer to this as a pure covalent bond. Chlorine, on the other hand, has an atomic number of 17 and has 7 electrons in its. Group 6a form 2 bonds; 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy only one. Web how many covalent bonds does chlorine form?
Covalent Bonding (Biology) — Definition & Role Expii
Web one, two, or three pairs of electrons may be shared between atoms, resulting in single, double, or triple bonds, respectively. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Web typically, the atoms of group 4a form 4 covalent bonds; In the case of cl 2, each atom starts off. And group 7a.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Web 4 rows the slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom,. The slideshow shows how a covalent bond forms between a hydrogen atom and a chlorine atom,. The order of bonding, and so the valence state of.
Is SiO2 Ionic or Covalent? Techiescientist
They end of sharing 6 electrons between the. So by sharing electrons through covalent bond formation, atoms are able to fill. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Web we refer to this as a pure covalent bond. The halogens all have the general electron configuration ns2np5, giving them seven valence.
Chlorine combined with two negative atom or 1 positive and other
And group 7a form one bond. Web cl2 +h2o → hocl + hcl (1) (1) c l 2 + h 2 o → h o c l + h c l at the boiling temperature of water, chlorine decomposes water: Group 6a form 2 bonds; Web the bond in a hydrogen molecule, measured as the distance between the two nuclei,.
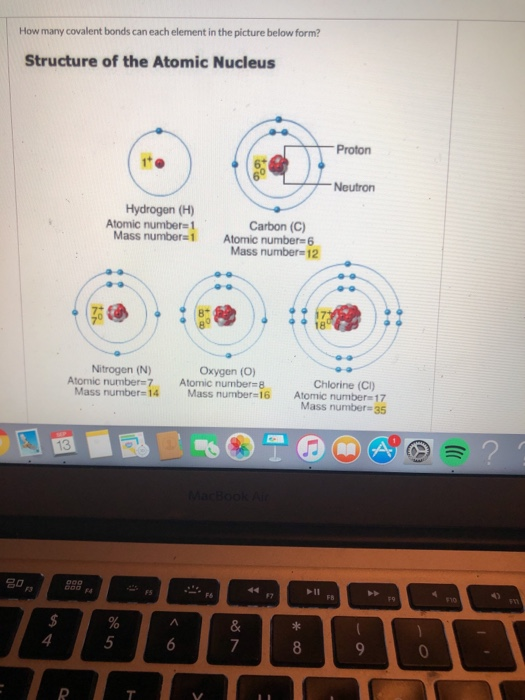
Solved How many covalent bonds can each element in the
Web sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. So by sharing electrons through covalent bond formation, atoms are able to fill. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; The more electrons.
Covalent Bond Biology Dictionary
Web we refer to this as a pure covalent bond. 1 pm = 1 × 10 −12 m). 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy only one. The order of bonding, and so the valence state of cl in cloxxx−, x > 1 c l o x x x −, x > 1.
How Many Covalent Bonds Can Calcium Form MaleahkruwJarvis
Web sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. So by sharing electrons through covalent bond formation, atoms are able to fill. Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for an octet. Electrons shared in pure.
Bonding A Level Notes
4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy only one. Web usually each atom contributes one electron to the shared pair of electrons. Web typically, the atoms of group 4a form 4 covalent bonds; If it shares one of those with another chlorine atom (and the other one with the first), they can both..
Web How Many Covalent Bonds Can Chlorine Form?
A chlorine atom has 7 electrons in its outer shell. The slideshow shows how a covalent bond forms between a hydrogen atom and a chlorine atom,. Web sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm;
1 Pm = 1 × 10 −12 M).
Web in chlorine an electron pair is shared between the two atoms in cl 2. So by sharing electrons through covalent bond formation, atoms are able to fill. Web we refer to this as a pure covalent bond. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus.
They End Of Sharing 6 Electrons Between The.
The halogens all have the general electron configuration ns2np5, giving them seven valence. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. Web usually each atom contributes one electron to the shared pair of electrons. The more electrons that are shared between two.
2Cl2 + 2H2O → 4Hcl +O2 (2).
And group 7a form one bond. Web cl2 +h2o → hocl + hcl (1) (1) c l 2 + h 2 o → h o c l + h c l at the boiling temperature of water, chlorine decomposes water: This is called covalent bonding. Web typically, the atoms of group 4a form 4 covalent bonds;